Abstract
Introduction Cytotoxic cutaneous T-cell lymphomas (CCTCLs) are an uncommon group of neoplasms defined by expression of at least one cytotoxic marker (e.g. TIA-1, granzyme B, or perforin). Due to their rarity, comparatively little is known about their presentation and overall course. Here, we present a detailed retrospective experience of patients with CCTCLs, focusing on their clinicopathologic and molecular characteristics, treatment details, and survival outcomes.
Methods The Stanford University Cutaneous Lymphoma Database was queried to gather data on patients with CCTCLs seen between 1995-2020, including the following diagnoses: subcutaneous panniculitis-like T-cell lymphoma (SPTCL), primary cutaneous gamma-delta T-cell lymphoma (PCGDTCL), primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (PCAECTCL), and unclassified CCTCLs (CCTCL-NOS). Clinical and laboratory data, including clinical suspicion of hemophagocytic syndrome (HPS), were extracted from the electronic medical record. Treatment data were recorded, including use of radiotherapy (RT), pralatrexate, romidepsin, brentuximab vedotin (BV), combination chemotherapy, allogeneic stem cell transplant (allo-SCT), and others. We evaluated time to next treatment (TTNT), calculated as the start date of one line of therapy to the start date of next line of therapy, in addition to overall survival (OS) and factors predictive of outcome. A subset of patients was evaluated with a clinically validated high-throughput sequencing panel for hematopoietic and lymphoid neoplasms (Heme-STAMP) covering 164 genes.
Results 46 patients were included: 22 (47.8%) SPTCL, 13 (28.2%) PCGDTCL, 3 (6.5%) PCAECTCL, and 8 (17.3%) CCTCL-NOS. Mean age at diagnosis was 55.7 years (range: 14.9 - 87.4). Most patients (87%) presented with generalized skin lesions, while involvement of lymph nodes (22.2%), viscera (8.9%), or bone marrow involvement (3.1%) were uncommon. Clinical suspicion for HPS was noted in 23 (50%) patients.
Median lines of therapy received was 2.5 (range: 1-12). Median TTNT (mTTNT) from first-line therapy was 8.5 months, second-line 5.3 months, and third-line 7.8 months. Variation in mTTNT was observed between CCTCL subtypes. In patients with SPTCL, mTTNT from first and second-line therapy were 10.2 and 24.0 months, respectively. In patients with PCGDTCL, mTTNT after first-line therapy was 11.1 months, decreasing sharply thereafter (0.5-3.1 mo. for 2nd-5th lines). Patients with PCAECTCL had short mTTNT of less than 3 months for all lines of treatment. Patients with CCTCL-NOS exhibited more heterogeneity (mTTNT 2.9 - 9.1 mo. for 1st-5th lines).
The most commonly used treatments were combination chemotherapy (n=24 instances, mTTNT 7.2 mo.), pralatrexate (n=18, mTTNT 11.6 mo.), romidepsin (n=17, mTTNT 2.4 mo.), RT (n=16, mTTNT 3 mo.), and BV (n=11, mTTNT 3.7 mo.). Median TTNT varied between CCTCL subtypes and was generally longest in patients with SPTCL.
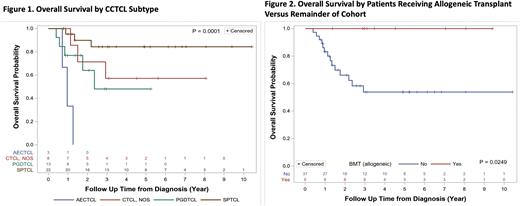
2- and 5-year OS were 73.5% and 64.2%, respectively. Significant differences were observed between CCTCL subtypes (Figure 1).Age > 60 was associated with worse survival (2-year OS 42.3% vs. 100%, p < .0001). Extracutaneous involvement was not associated with outcome (2-year OS 60.6% vs. 69.1%, p=0.5), nor was documented clinical suspicion for HPS (2-year OS 82.2% vs. 64.8%, p=0.21). Patients undergoing allo-SCT (n=9) had superior OS (2-year OS 100% vs. 66.1%, p=0.03) (Figure 2).
Thirteen (28.3%) patients had data available from Heme-STAMP testing; every patient who underwent testing had at least one identified mutation. The most frequently occurring somatic mutations included TP53 (n=3), TERT (n=3), and JAK1 (n=3).
Conclusions CCTCLs are rare entities with heterogeneous presentation, response to therapy, and survival. Chemotherapy remains an effective treatment, and we identify pralatrexate as a promising single agent option. Allo-SCT offers curative potential in eligible patients. Given wide variation in disease behavior, treatment intensity should be tailored to each individual's clinical phenotype to optimally balance risk and benefit. Further efforts to define the molecular basis of CCTCLs are urgently needed to aid in the identification of more effective therapies.
Disclosures
Fernandez-Pol:Cartography Biosciences: Consultancy; Leica Biosystems: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees. Kim:Kyowa Kirin: Honoraria, Research Funding; Eisai: Research Funding; INNATE PHARMA: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Honoraria; Secura Bio: Honoraria; Trillium: Research Funding; Galderma: Honoraria, Research Funding; Mundipharma: Honoraria; Elorac: Research Funding; Sanofi: Honoraria; CRISPR Therapeutics: Research Funding; Corvus: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola/Alexion Pharma: Research Funding; Soligenix: Research Funding. Khodadoust:Nutcracker Therapeutics: Research Funding; CRISPR Therapeutics: Research Funding; Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.